
- •Dedication
- •Preface
- •Acknowledgments
- •Figure Credits
- •Expert Consultants and Reviewers
- •Contents
- •Descriptive Terms for Normal Cells
- •Descriptive Terms for Abnormal Cells and Tissues
- •Epithelium
- •Glands
- •Introduction and Key Concepts for Connective Tissue
- •Cartilage
- •Bone
- •Introduction and Key Concepts for the Nervous System
- •Peripheral Blood Cells
- •Hemopoiesis
- •Introduction and Key Concepts for the Circulatory System
- •The Cardiovascular System
- •Introduction and Key Concepts for the Lymphoid System
- •Cells in the Lymphoid System
- •Introduction and Key Concepts for the Respiratory System
- •Conducting Portion
- •Respiratory Portion
- •Introduction and Key Concepts for the Urinary System
- •Introduction and Key Concepts for the Integumentary System
- •Oral Mucosa
- •Teeth
- •Introduction and Key Concepts for the Digestive Tract
- •Introduction and Key Concepts for the Endocrine System
- •Introduction and Key Concepts for the Male Reproductive System
- •Introduction and Key Concepts for the Female Reproductive System
- •Introduction and Key Concepts for the Eye
- •Introduction and Key Concepts for the Ear
- •Introduction
- •Preservation versus Fixation
- •Fixatives and Methods of Fixation
- •Sectioning and Mounting
- •Staining
- •Index

CHAPTER 8 ■ Blood and Hemopoiesis |
145 |
Hemopoiesis
Introduction and Key Concepts for Hemopoiesis
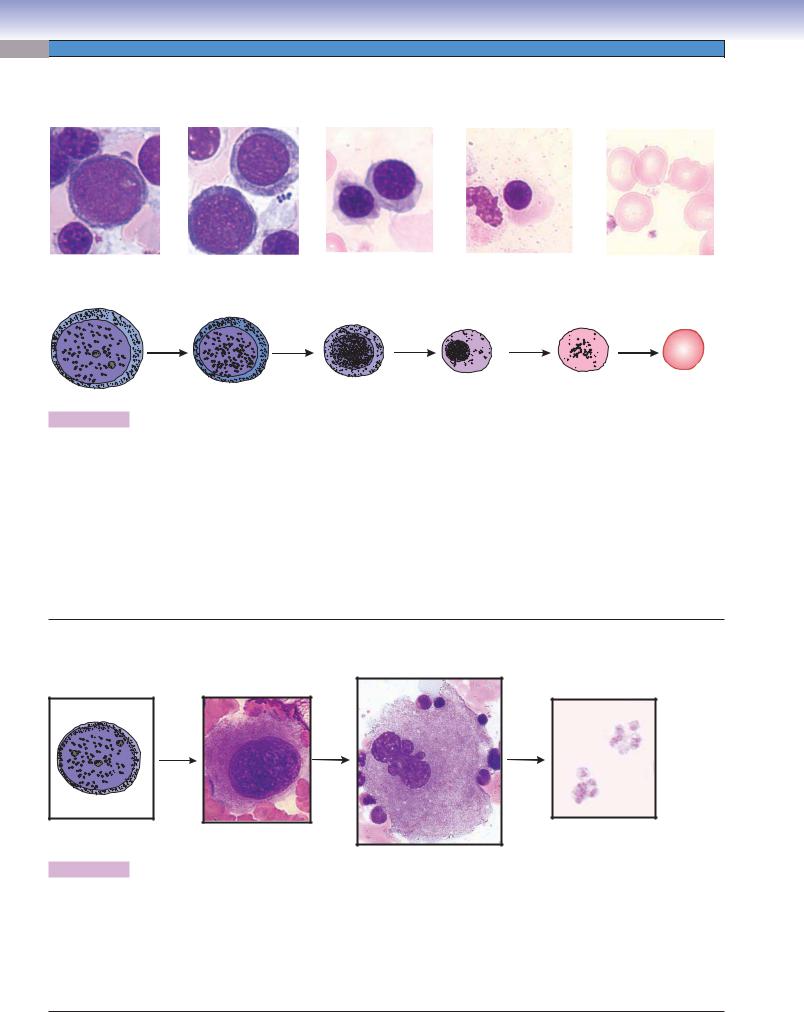
All formed elements, with the exception of some lymphocytes, have a finite life span in circulation, so there must be an ongoing replacement throughout the life of an individual. The magnitude of the task for a given cell type can be appreciated if the approximate required daily production rate is calculated from estimates of the total number in circulation and the rate of turnover for the cell type. For erythrocytes, with a life span of about 120 days, the daily production rate is roughly 250 billion, for neutrophils, with a time in circulation of less than a day, the daily production rate is normally roughly 60 billion, and for platelets, with a life span of about 10 days, the daily production rate is approximately 150 billion. The development of each type of blood cell involves numerous cell divisions and a series of differentiation steps so that a small number of completely undifferentiated stem cells produce enormous numbers of cells that have the specific equipment necessary for the particular mature cell to perform its functions. Although all blood cells originate from a common pluripotential hemopoietic stem cell, each blood cell type has its own lineage of cell generations committed to proliferate and, at the same time, differentiate only into that cell type. Cells that can be recognized morphologically as undertaking differentiation into a particular blood cell are called precursor cells. Precursor cells are produced by cells that are committed to a specific lineage (i.e., they are determined to give rise to, e.g., only erythrocytes) but show no morphological signs of differentiation. These are called progenitor cells. Some progenitor cells are not restricted in potential to just one blood cell lineage but rather to two lineages. Progenitor cells are also termed colony-forming cells (CFCs). A commonly used abbreviation system for designating specific progenitor cells uses the first letter of the blood cell name after the letters CFC, for example, CFC-E for erythrocyte colony–forming cell and CFC-B for basophil colony–forming cell. Development of blood cells occurs mostly in the specialized environment of the bone marrow. Because extensive cell proliferation is required, the process is very vulnerable to irradiation, so protection within the cores of bones is clearly advantageous. The development of each type of blood cell involves a series of precursor cells that can be recognized in smears of the red bone marrow that have been stained with the same procedures used for peripheral blood smears. Lymphocytes and monocytes are little differentiated, so the morphological appearance of their precursors (lymphoblasts and promonocytes, respectively) is not easily distinguished. By contrast, the precursors of erythrocytes, granulocytes, and platelets exhibit relatively distinct features as they differentiate in a series of steps that involve predictable changes and identifiable stages. The red bone marrow is a hemopoietic compartment where blood cells (except lymphocytes) develop and mature (Fig. 8-15C).
ERYTHROCYTE DEVELOPMENT is called erythropoiesis. The appearances of the precursors of erythrocytes reflect the processes that must take place to generate, from an undifferentiated cell, a cell that is essentially a plasmalemma bag of hemoglobin. In the initial stage, the proerythroblast, the main event is generation of free ribosomes that will be needed to synthesize the globin that will combine with heme to form hemoglobin. Therefore, the intense basophilic staining of the cytoplasm in the next stage, the basophilic erythroblast, results from a peak concentration of free ribosomes that begin translation of globin mRNAs. In the polychromatophilic
erythroblast, enough hemoglobin has accumulated to confer some eosinophilia to the cytoplasm, whereas the concentration of ribosomes has decreased from dilution that accompanies cell division. Continuation of cell division, dilution of ribosomes, and accumulation of more hemoglobin account for the strong eosinophilic staining of the cytoplasm in the orthochromatophilic erythroblast
(normoblast). Concurrent with the successive changes in staining of the cytoplasm during erythrocyte development in the cytoplasm are changes in the appearance of the nucleus. Production of ribosomes and transcription of mRNA for globin and other proteins are manifested by a large euchromatic nucleus with prominent nucleoli in the proerythroblast; subsequent stages have progressively smaller, less active nuclei, and the nucleus is ultimately extruded at the end of the orthochromatophilic erythroblast stage. The mitochondrion, another key organelle, is required to synthesize protoporphyrin and combine it with iron to form the heme of hemoglobin (Figs. 8-9A and 8-10A to 8-11C).
PLATELET DEVELOPMENT is called thrombopoiesis. Although platelets are small (2–4 μm in greatest diameter) fragments of highly organized cytoplasm, they are produced by very large cells called megakaryocytes. These cells measure up to 100 μm or more in diameter. Megakaryocytes develop from precursor cells (called megakaryoblasts) through a series of incomplete cell cycles (endomitosis) that do not include division of the nucleus or cytoplasm. The result is that the nucleus of a mature megakaryocyte has up to 64N chromosomes, instead of the usual 2N chromosomes. The nucleus is large and lobular, but it remains one nucleus. The cytoplasm develops numerous mitochondria, a variety of granules, and microfilaments and microtubules. As the megakaryocyte reaches maturity, its cytoplasm becomes cordoned off by an elaborate system of membranes, called demarcation membranes or channels, which subdivide the cytoplasm into platelet zones—like perforations in a sheet of stamps (Figs. 8-9B and 8-12A–C).
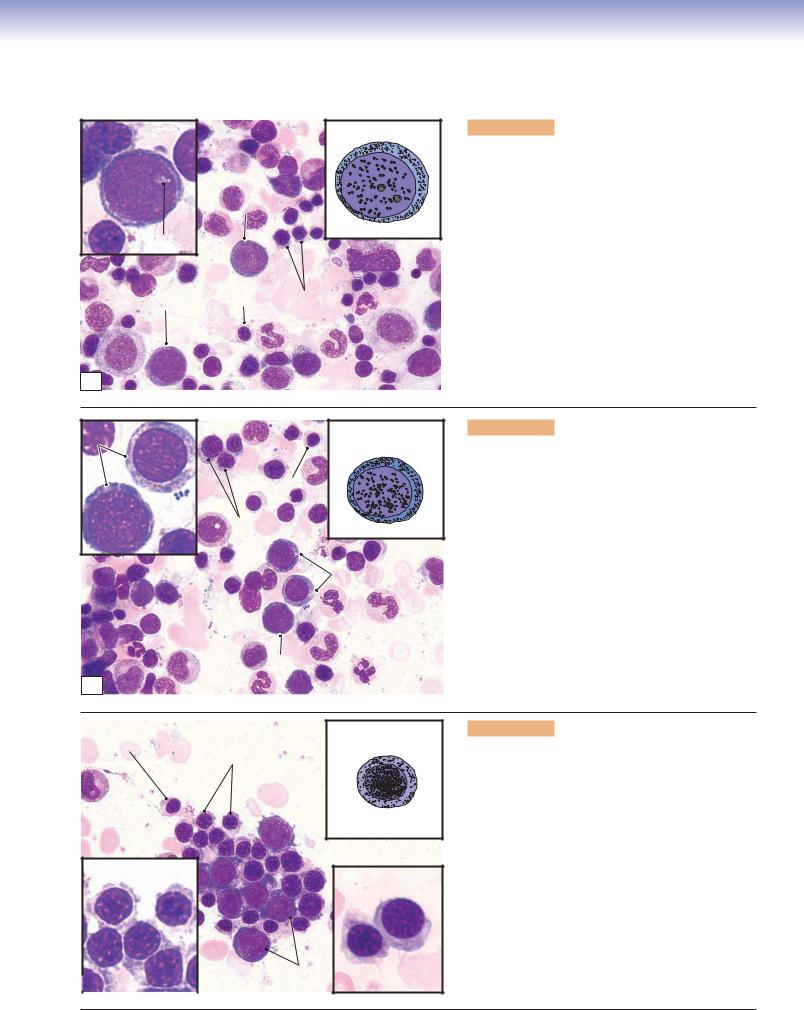
GRANULOCYTE DEVELOPMENT (GRANULOCYTOPOIESIS) proceeds through an orderly sequence of events that result in cytoplasm that is packed with granules containing a wide variety of substances related to inflammation and destruction of pathogenic organisms. As is the case with erythrocyte development, the initial discernible event is generation of components (ribosomes and RNA) needed for protein synthesis, but in granulocyte development, the proteins will be packaged in vesicles (granules). Accordingly, packaging of the proteins requires development of an extensive endoplasmic reticulum and Golgi complex. These events are prominent in the myeloblast and promyelocyte stages, both of which have relatively large, active nuclei with nucleoli and cytoplasm that is basophilic owing to its content of ribosomes. Granule generation occurs sequentially, the nonspecific (lysosomal) granules first, in the promyelocyte stage, and the specific granules second, in the myelocyte stage. Because specific granules first appear in the myelocyte stage, this is the earliest stage at which the precursors of the three granulocytes can be distinguished from each other. Other notable changes during granulocyte maturation are progressive condensation, elongation, and segmentation of the nucleus (Figs. 8-13A to 8-15B).
146 UNIT 2 ■ Basic Tissues
A
Proerythroblast |
|
Basophilic |
|
Polychromatophilic |
|
Orthochromatophilic |
Erythrocytes |
|
|
erythroblast |
|
erythroblast |
erythroblast (normoblast) |
||||
|
|
|
|
|
|
|
|
|
Proerythroblast |
Basophilic |
Polychromatophilic |
Orthochromatophilic |
|
|
erythroblast |
Reticulocyte |
Erythrocytes |
|||
|
erythroblast |
erythroblast |
(normoblast) |
|
|
D. Cui
Figure 8-9A. A representation of erythropoiesis (red blood cell formation), bone marrow. Wright stain, 1,569
Erythrocyte formation includes several stages of cell changes during differentiation. Erythrocytes derive from progenitor cells (CFC-Es) that give rise to the first recognizable erythrocyte precursor, the proerythroblast. The proerythroblast is a large cell, which has a large active nucleus with nucleoli. Each proerythroblast divides into two basophilic erythroblasts. Each basophilic erythroblast divides into two polychromatophilic erythroblasts, each of which then divides to form orthochromatophilic erythroblasts, which do not divide. These, in turn, differentiate into reticulocytes (Fig. 8-11B,C), which finally become erythrocytes. There are some general tendencies accompanying differentiation of erythrocytes: (1) the overall size of the cells decreases, (2) the nucleus size decreases and the condensation of the chromatin increases, (3) nucleoli disappear, and (4) the color of the cytoplasm changes from blue to gray to pink because of a reduction of ribosomes and an increase of hemoglobin. When the ribosomes are diluted by cell division and the hemoglobin concentration rises to a near mature level, the cell becomes an orthochromatophilic erythroblast (or normoblast). When the nucleus is extruded and only a few organelles (polyribosomes and mitochondria) remain in the cytoplasm, the cell is called a reticulocyte. The reticulocyte completes maturation and enters the blood circulation to become a mature erythrocyte (red blood corpuscle).
B
Megakaryocyte
Megakaryoblast |
Promegakaryocyte |
Platelets |
D. Cui
Figure 8-9B. Thrombopoiesis (platelet formation process), bone marrow. Wright stain, 843, 586, and 1,570 (from left to right)
Platelets are very small fragments of cells that have no nuclei. They are also called thrombocytes. Their differentiation from a large cell, the megakaryocyte, takes place in the bone marrow. Megakaryoblasts are the precursor cells. They have a large, round nucleus, undergo mitosis, and become promegakaryocytes. These cells have a large, round nucleus and develop through growth and a series of endomitoses into megakaryocytes. A megakaryocyte has a large, multilobed nucleus with a huge amount of cytoplasm containing numerous granules. The maturation process includes the development of a demarcation membrane system and the subdivision of the cytoplasm to form platelets (Fig. 8-12A,B).
CHAPTER 8 ■ Blood and Hemopoiesis |
147 |
Erythropoiesis
Proerythroblast |
Proerythroblast |
Proerythroblast
D. Cui
Nucleoli
Proerythroblast |
OE |
PoE |
A
Figure 8-10A. Proerythroblasts, bone marrow smear.
Wright stain, 710; inset 1,569
The proerythroblast is a relatively large cell with a large, round nucleus containing two to three nucleoli. The cytoplasm appears basophilic (blue) because of the presence of a large number of ribosomes. At this stage, the cell is beginning to accumulate the necessary components for the production of hemoglobin. Proerythroblasts are precursor cells, which develop from two functionally identifiable progenitor cells: burst-forming unit–erythroid (BFU-E) cells, which take about a week to mature to become colony-forming unit–erythroid
(CFU-E) cells and another week to become proerythroblasts. Proerythroblasts undergo mitosis to produce two daughter cells that will develop the features of basophilic erythroblasts.
BE |
Basophilic |
|
erythroblast |
||
|
||
|
OE |
|
PoE |
D. Cui |
|
|
Basophilic |
|
|
erythroblast |
Basophilic erythroblast
B
Figure 8-10B. Basophilic erythroblasts, bone marrow smear. Wright stain, 710; inset 1,569
The basophilic erythroblast is smaller than the proerythroblast, and its cytoplasm is deep blue because of a high content of tightly packed ribosomes. Compared to proerythroblasts, basophilic erythroblasts have smaller nuclei with a coarser texture because most of the chromatin is in the heterochromatin form. At this stage, nuclei are less active than in proerythroblasts, and their nucleoli disappear. These cells undergo mitosis and divide into daughter cells, which mature to become polychromatophilic erythroblasts. (OE, orthochromatohilic erythroblast; PoE, polychromatophilic erythroblast; BE, basophilic erythroblast.)
Orthochromatophilic |
|
Polychromatophilic |
erythroblast |
Polychromatophilic |
erythroblast |
|
erythroblasts |
|
D. Cui
Polychromatophilic
erythroblast Polychromatophilic erythroblast
|
|
Basophilic |
C |
|
erythroblast |
Figure 8-10C. Polychromatophilic erythroblasts, bone marrow smear. Wright stain, 710; inset 1,569
The polychromatophilic erythroblast is smaller than its parent cell (basophilic erythroblast). The nucleus is smaller and it has no nucleoli. The condensed nucleus is densely stained, and it displays a patchy pattern because of condensation of chromatin. The cytoplasm of the cell is usually grayish in overall color because, at this stage, significant amounts of hemoglobin have been produced and accumulated in the cytoplasm, so that the staining of the cytoplasm reflects the presence of both ribosomes (basophilic) and hemoglobin (eosinophilic). Therefore, the cytoplasm is mottled with mixed patches of blue and pink (polychromatophilic means “attracting multiple colors”). Polychromatophilic erythroblasts undergo mitosis and divide into daughter cells that develop into orthochromatophilic erythroblasts.

148 UNIT 2 ■ Basic Tissues
A |
PoE |
Orthochromatophilic |
Figure 8-11A. Orthochromatophilic erythroblasts, bone |
|
marrow smear. Wright stain, 710; inset 1,569 |
||||
|
erythroblast |
|||
|
|
|
||
|
|
|
The orthochromatophilic erythroblast, also called a |
|
|
|
|
normoblast, is a very small cell, close to the size of an |
|
PoE |
|
|
erythrocyte. The nucleus is small and so condensed that |
|
|
D. Cui |
it looks like a dark dot because of the extreme condensa- |
||
|
|
|
tion of the chromatin. The cytoplasm appears pinker than |
|
|
|
|
that of the polychromatophilic erythroblast. Hemoglobin |
|
|
|
Orthochromatophilic |
production and accumulation are almost complete, with |
|
|
|
few ribosomes left in the cytoplasm. At this stage, the cell |
||
|
|
erythroblast |
||
|
|
|
is unable to divide. Orthochromatophilic erythroblasts |
|
|
Proerythroblast |
|
become reticulocytes (Fig. 8-11B) after losing their nuclei. |
|
|
|
|
||
OE |
|
|
|
|
B |
|
|
Figure 8-11B. Reticulocytes: the final step of erythro- |
|
Orthochromatophilic |
|
|
cyte formation. |
|
|
|
|
||
erythroblast |
|
Reticulocyte |
|
|
(normoblast) |
|
Orthochromatophilic erythroblasts have small and highly |
||
|
|
|||
|
|
|
condensed nuclei. In the next stage, the nucleus is extruded |
|
|
|
|
and phagocytosed by macrophages. Although the cells |
|
|
|
|
loose their nuclei, they retain some polyribosomes in their |
|
|
|
|
cytoplasm. When stained supravitally with cresyl blue or |
|
Mature |
|
|
new methylene blue, the ribosomes aggregate into a blue |
|
|
|
reticular network; therefore, the cells are called reticulo- |
||
erythrocyte |
|
|
cytes. Reticular cells appear the same as mature erythro- |
|
(inside of capillary) |
|
|
cytes with Wright stain (Fig. 8-11C). Reticulocytes enter |
|
|
|
|
||
|
|
|
the blood circulation through the bone marrow sinusoidal |
|
|
|
|
capillaries and become mature erythrocytes in one or two |
|
|
|
|
days. Mature erythrocytes have neither nuclei nor organ- |
|
|
|
|
elles and appear as a biconcave disk. |
|
D. Cui |
|
|
|
|
|
Capillaries |
|
|
CLINICAL CORRELATION
C
Erythrocyte
 Reticulocytes
Reticulocytes
Figure 8-11C. Reticulocytosis, Peripheral Blood Smear. New methylene blue stain, 1,020
Reticulocytosis is a condition characterized by an increased number of reticulocytes. Reticulocytes are premature red blood cells. The normal percentage of reticulocytes is 0.5% to 1.5%. Hemolytic anemia usually increases erythropoietin production, which in turn causes the bone marrow to produce more red blood cells, resulting in a reticulocyte percentage of above 4% to 5%. An increased number of reticulocytes in peripheral blood is an important indication of hemolysis (red blood cell rupture) or bleeding. It can also be the consequence of treating the anemia of chronic kidney disease with erythropoietin. This illustration shows the increased number of reticulocytes with new methylene blue stain after hemolytic anemia.

CHAPTER 8 ■ Blood and Hemopoiesis |
149 |
Thrombopoiesis
A
Megakaryoblast
Figure 8-12A. Promegakaryocytes (immature megakaryocytes), bone marrow smear. Wright stain, 754; inset 1,605
Promegakaryocytes develop from megakaryoblasts in the bone marrow. Megakaryoblasts lose their ability to undergo cytokinesis but undergo a series of incomplete cell cycles called “endomitosis” that results in replication of DNA up to 64N without division of nuclei or cytoplasm. Each megakaryoblast has a large nucleus, multiple nucleoli, and basophilic cytoplasm (Fig. 8-9B). Promegakaryocytes can be recognized by their large size, round (or oval) nuclei, and large amount of cytoplasm. Development of a demarcation membrane system is an important feature of thrombopoiesis and begins at a very early stage. The demarcation membrane system is produced by the invagination of plasma membranes to form branched interconnected channels through the cytoplasm. This system may play an important role in later subdivision of the cytoplasm into platelet zones.
B |
Nucleus of the |
Incipient |
|
megakaryocyte |
platelets |
|
|
|
Neutrophils
Figure 8-12B. Megakaryocytes, bone marrow smear. Wright stain, 754; inset 2,827
Because of their very large size, megakaryocytes are sometimes called giant cells. They have a large indented (partially lobulated) nucleus and an extremely voluminous cytoplasm containing a variety of granules. In this stage, the demarcation membrane system is complete, and the megakaryocytes are ready to release platelets. Megakaryocytes tend to move toward and attach to the marrow sinusoids (capillaries in the bone marrow) after they become mature. The platelets are released into the blood circulation through the wall of the marrow sinusoids. Note the neutrophils on the surface of the megakaryocyte for size comparison. The inset shows incipient platelets (fragments of cytoplasm beginning to become platelets) in the surface region of the megakaryocyte that are ready to be released. Thrombopoietin, a humoral factor produced in the liver, is believed to regulate megakaryocytes and the production of platelets.
CLINICAL CORRELATION
C
Megakaryocytes
 Megakaryocyte
Megakaryocyte
Figure 8-12C. Essential Thrombocytosis, Bone Marrow Smear. Wright stain, 304
Essential thrombocytosis, also called essential thrombocythemia, is one of the myeloproliferative disorders, characterized by overproduction of platelets by megakaryocytes in the bone marrow without an identifiable cause. The platelet counts exceed 600,000/μL, but the platelets do not function properly. Symptoms and signs include headache; bleeding from gums, nose, and gastrointestinal tract; throbbing and burning pain of the hands and feet caused by thrombosis of small arterioles; and splenomegaly (enlarged spleen). Treatment includes using low-dose aspirin to control headache and other vasomotor symptoms and anticancer agents such as hydroxyurea to maintain proper platelet count. Bone marrow smears show increased numbers of megakaryocytes. Large platelets, similar in size to red blood cells, may be found in the peripheral blood.

150 UNIT 2 ■ Basic Tissues
Granulocytopoiesis
A
Myeloblast |
Neutrophilic |
Neutrophilic |
Neutrophilic |
Mature |
|
myelocyte |
metamyelocyte |
stab cell |
neutrophil |
Eosinophilic |
Eosinophilic |
Eosinophilic |
Mature |
myelocyte |
metamyelocyte |
stab cell |
eosinophil |
Promyelocyte
Basophilic |
Basophilic |
Basophilic |
Mature |
myelocyte |
metamyelocyte |
stab cell |
basophil |
D. Cui
Figure 8-13A. A representation of granulocytopoiesis.
In addition to erythropoiesis, leukopoiesis also occurs in the bone marrow. Here are examples of the development of granular leukocytes (granulocytopoiesis). The myeloblast is the earliest morphologically recognizable precursor cell. Cell division occurs in myeloblasts, promyelocytes, and myelocytes. The myelocyte is the last stage that is capable of dividing. The generation of nonspecific granules occurs in the promyelocyte stage and specific granules in the myelocyte stage. Maturation of granulocytes follows this sequence: myeloblasts, promyelocytes, myelocytes, metamyelocytes, and stab (band) cells. The following morphologic changes occur during granulocyte maturation: (1) nucleoli are present only before and during the promyelocyte stage; (2) the nucleus takes the following shapes in different developmental stages: oval, elongated, indented, arched, and then segmented (lobed); and (3) specific granules are first present at the myelocyte stage.
B
Neutrophilic |
Neutrophilic |
Neutrophilic |
Neutrophil |
myelocyte |
metamyelocyte |
stab cell |
Promyelocyte
Eosinophilic |
Eosinophilic |
Eosinophilic |
Eosinophil |
|||
myelocyte |
metamyelocyte |
stab cell |
||||
|
|
|
|
|
|
|
Figure 8-13B. Overview of stages of granulocytes in development, bone marrow smear. Wright stain, 1,569
These are examples of microphotographs that show various stages of granulocyte maturation in the neutrophilic and eosinophilic series.

CHAPTER 8 ■ Blood and Hemopoiesis |
151 |
Promyelocyte
Promyelocyte
D. Cui
Promyelocyte
Figure 8-14A. Promyelocytes, bone marrow smear. Wright stain, 710; inset 1,569
Promyelocytes (also called progranulocytes) have a round or oval-shaped nucleus with one to three nucleoli. The cytoplasm is light blue, containing some dark purple granules (azurophilic granules). At this stage, specific granules have not been produced. Promyelocytes vary in size and are produced by the division of myeloblasts. Their nuclei have a smooth, fine texture because of the fact that most of the chromatin is euchromatin, which is delicately dispersed.
Promyelocytes divide to form myelocytes.
A
Neutrophilic |
Neutrophilic |
Neutrophilic myelocyte |
myelocyte |
myelocyte |
|
|
|
D. Cui |
|
|
|
|
|
|
Eosinophilic |
|
|
myelocyte |
|
Eosinophilic myelocyte |
|
|
|
Eosinophilic |
|
|
|
myelocyte |
|
B |
|
D. Cui |
|
|
|
|
|
|
|
|
Figure 8-14B. Myelocytes, bone marrow smear. Wright stain, 710; inset 1,569
The myelocyte has an oval or kidney-shaped nucleus that has no nuclei and a coarse texture because of increasing heterochromatin content. The cytoplasm contains azurophilic granules and specific granules. In this stage, the cell has stopped producing azurophilic granules. In addition, these granules have also been diluted during cell division, so they are not as prominent as in the promyelocyte stage. At the same time, the cell has begun to make and accumulate specific granules so that the cytoplasm starts to take the characteristic appearance of a mature granulocyte. The specific granules in neutrophilic myelocytes are small and appear pinkish, granules in eosinophilic myelocytes are large and stain red, and granules in basophilic myelocytes are blue-purple. Basophilic myelocytes are least numerous and, therefore, difficult to find. In general, myelocytes are smaller than promyelocytes, and their nuclear shape and size may vary. This stage is the longest in granulocytopoiesis.
Neutrophilic |
Neutrophilic |
metamyelocyte |
metamyelocyte |
D. Cui
Eosinophilic metamyelocyte
|
|
|
Eosinophilic |
|
|
|
|
metamyelocyte |
Neutrophilic |
|
|
|
|
metamyelocyte |
|
C |
|
|
|
|
|
|
|
Figure 8-14C. Metamyelocytes, bone marrow smear.
Wright stain, 710; inset 1,569
Metamyelocytes are small cells. They have condensed nuclei, which are elongated with various degrees of indentation and contain clumped chromatin. Metamyelocytes are unable to divide (myelocytes are the last stage in cell division). The cytoplasm of metamyelocytes contains both types of granules. Their cytoplasm and granules are similar to those of mature granulocytes. At this stage, cells have their distinguishing features, but their nuclei have not yet become segmented.

152 UNIT 2 ■ Basic Tissues
Neutrophilic |
Neutrophilic Neutrophilic stab cells |
Figure 8-15A. Stab (band) cells, bone marrow smear. Wright |
|
stab cell |
stab cell |
|
stain, 710; insets 1,569 |
|
|
|
Granular metamyelocytes mature to become stab cells, which |
|
Eosinophilic |
|
are also called band cells. The stab cells (mainly neutrophilic |
|
|
stab cells) can be found in both the bone marrow and the |
|
|
myelocyte |
|
|
|
D. Cui |
|
peripheral blood. Their nuclei are elongated and become band |
|
Eosinophilic |
|
and arch (or “C”) shaped, and the cytoplasm is the same as |
Eosinophilic |
metamyelocyte |
|
that of mature neutrophils. These cells are the last stage of |
|
|
granulocyte maturation without division, and in function and |
|
stab cell |
Basophilic |
|
|
|
|
structure, they are very close to mature neutrophils. The nuclei |
|
|
erythroblast |
|
of mature neutrophils become multilobulated (segmented), |
|
|
|
|
|
|
|
contain dense heterochromatin, and often are described as |
|
|
|
polymorphonuclear (or segmented) neutrophils. |
A |
Neutrophilic |
Neutrophilic |
|
myelocyte |
|
||
stab cell |
|
||
Orthochromatophilic |
Polychromatophilic |
Neutrophilic |
erythroblast |
erythroblasts |
|
|
|
metamyelocyte |
Eosinophil
|
|
Neutrophil |
Neutrophilic |
|
|
|
|
|
Proerythroblast |
|
stab cell |
B |
|
||
|
|
|
Figure 8-15B. Bone marrow cells, bone marrow smear. Wright stain, 710
This is an example of blood cells at various stages of development in the bone marrow, which includes both the erythrocyte and the granulocyte series. These cells, at various stages of development, are densely packed together and can be found randomly distributed in the bone marrow. During the maturation process, the cell size becomes smaller and nuclei become denser. In the erythropoiesis series, the cytoplasm of cells becomes light blue and then more pink, and nuclei become much denser and smaller and finally disappear. In the granulocytopoiesis series, the cytoplasm becomes less blue, primary (nonspecific) granules are produced, and then specific granules are produced and are present in myelocytes, which gives these cells the appearance characteristic of their identity as a neutrophil, eosinophil, or basophil. In other changes, nuclei become progressively denser, and the shape changes from round to oval, elongated, indented, and then lobed (segmented).
Blood cells
Reticular tissue
Adipocyte
spaces
C
Figure 8-15C. Bone marrow, bone marrow smear. Wright stain, 35; inset 184
Bone marrow is a specialized example of a reticular connective tissue, a loose connective tissue in which numerous cells are supported by a delicate network of reticular fibers. It resides in cavities within bones (see Figs. 5-8, 5-10, and 5-11). Bone marrow can be categorized into red bone marrow and yellow bone marrow. The term red bone marrow denotes active hematopoiesis; yellow bone marrow refers to a marrow composed chiefly of adipocytes (fat cells). Pictured is a smear of red bone marrow, which contains many developing blood cells, a few adipocytes, and some thin-walled blood vessels (sinusoidal capillaries). The red bone marrow is organized into a hemopoietic compartment and a vascular compartment. The hemopoietic compartment is a network of reticular fibers in which immature and mature blood cells are suspended. The vascular compartment is composed of mainly sinusoidal capillaries, which allow mature blood cells to enter the blood circulation.

CHAPTER 8 ■ Blood and Hemopoiesis |
153 |
Mature neutrophili
or neutrophilic Hematopoietic metamyelocyte compartment
Sinusoidal
capillary
Leukocyte granules
Sinusoidal
capillary
Endothelial cell
 Leukocyte precursors
Leukocyte precursors
Hematopoietic
compartment
Figure 8-16. Developing blood cells in the bone marrow. EM, 5,000
The two compartments of the red (hematopoietically active) bone marrow can be distinguished here. The vascular compartment is composed of sinusoidal capillaries, which in this view contain numerous mature erythrocytes. The hematopoietic compartment is composed of blood cells and the precursors and progenitors of blood cells suspended in a loose network of support cells and reticular fibers. The cells seen in the hematopoietic compartment here appear to be mostly developing or mature granulocytes. The exact stage of differentiation of individual cells is not as clear here as it is in a bone marrow smear.

154 UNIT 2 ■ Basic Tissues
SYNOPSIS 8 - 2 Hematopoiesis
Stem, Progenitor, and Precursor cells
■Stem cells are capable of differentiating into multiple cell lineages and can undergo proliferation indefinitely.
■Progenitor cells are only capable of differentiating into a single cell lineage (restricted to one or two blood cell types) and are morphologically undifferentiated.
■Precursor cells can be recognized morphologically as undergoing differentiation along a particular blood cell lineage.
Erythropoiesis
■Cytoplasm becomes progressively less basophilic because of dilution of ribosomes during the erythropoiesis process.
■Nucleus size progressively decreases because of increased condensation of chromatin.
■Cell size progressively decreases during the erythroid differentiation.
■Cytoplasm becomes progressively more eosinophilic because of increased accumulation of hemoglobin.
■The nucleus retains a round shape and no indentation occurs before it disappears.
Granulocytopoiesis
■Cell size decreases and nucleus becomes more condensed as in erythropoiesis.
■Nucleus shape changes from round or oval (promyeloblasts) to kidney shaped/slightly indented (myelocytes) and then changes from deeply indented (metamyelocytes) to band shaped (band cells) and finally to lobed (mature granulocytes).
■Promyelocytes do not have specific granules (only azurophilic granules); at this stage, it is too early to tell which granular leukocytes they will become.
■Myelocytes are the last developmental stage capable of dividing; specific granules accumulate in this stage.
SYNOPSIS 8 - 3 Pathological and Clinical Terms for Mature and Developing Blood Cells
■Gray platelet syndrome: This condition is characterized by a deficiency or absence of the alpha granules and contents in blood platelets, giving platelets a gray appearance in a Wright stain smear (Fig. 8-2B).
■Platelet storage pool deficiency: Disorder caused by a decrease or absence of platelet delta granules (dense bodies), which normally store and release adenine nucleotides and 5HT. “Platelet-type” bleeding is common with this deficiency (Fig. 8-2B).
■Petechiae: Minute red or purple spots on the skin or mucous membranes caused by capillary hemorrhage; common causes include physical trauma and decreased platelets (thrombocytopenia).
■Smudge cell: Damaged lymphocytes seen on a peripheral blood smear caused by mechanical stress in the process of producing the smear; although nonspecific, smudge cells are encountered more frequently on blood smears of patients with chronic lymphocytic leukemia (Fig. 8-4C).
■Reticulocytosis: Increased reticulocytes in the blood, often in response to blood loss, stimulation by erythropoietin treatment, or treatment of iron deficiency anemia with iron supplementation (Fig. 8-11C).
■Thrombocytosis: Increased platelet count in the blood, which may be reactive or neoplastic, as in the disease essential thrombocytosis (Fig. 8-12C).

UNIT 3 ■ Organ Systems
9 Circulatory System
Introduction and Key Concepts for the Circulatory System
The Cardiovascular System
Figure 9-1 |
Overview of the Cardiovascular System |
Figure 9-2 |
The Heart and Its Impulse Conductive Function |
Synopsis 9-1 |
Structure and Functions of the Heart |
Figure 9-3A |
Layers of the Heart Wall: Endocardium, Ventricle |
Figure 9-3B |
Layers of the Heart Wall: Myocardium, Ventricle |
Figure 9-3C |
Layers of the Heart Wall: Epicardium, Ventricle |
Figure 9-4A |
Purkinje Fibers and Intercalated Disks |
Figure 9-4B |
Cardiac Valves |
Figure 9-4C |
Clinical Correlation: Myocardial Infarction |
Figure 9-5 |
A Representation of the General Structure of Blood Vessel Layers (Tunicae) and a |
|
Comparison of the Medium Artery and the Medium Vein |
Figure 9-6 |
A Representation of Types of Blood Vessels: Arteries, Veins, and Capillaries |
The Arterial System |
|
Figure 9-7A–C |
Large Arteries (Elastic Arteries) |
Figure 9-8A–C |
Medium Arteries (Muscular Arteries) |
Figure 9-9A |
Medium Artery |
Figure 9-9B |
Small Artery |
Figure 9-10A–C |
Small Arteries and Arterioles |
Figure 9-11 |
Arteriole |
Synopsis 9-2 |
Functions of Endothelium in Blood Vessels |
Figure 9-12A |
Clinical Correlation: Coronary Artery Atherosclerosis |
Figure 9-12B |
Clinical Correlation: Polyarteritis Nodosa (Vasculitis) |
Synopsis 9-3 |
Pathological and Clinical Terms for the Circulatory System |
155
